How Does a Water Softener Work?
By Thomas Klenck
It makes hard water easy to get along with.
It’s easy to forget how important water is in our lives. Of course we need it in our diet, but in our homes, it’s a tool — a fluid medium that carries material from one place to the next. And one of the reasons it does this job well is that it’s very good at holding things, either by suspending them or dissolving them.
Unlike most tools, though, water doesn’t come with an instruction manual. If it did, you’d know why the dishes you thought were washed are covered with spots when dry, why the water in your shower leaves a film on everything it touches, and why what you thought was clean water has clogged up your plumbing system.
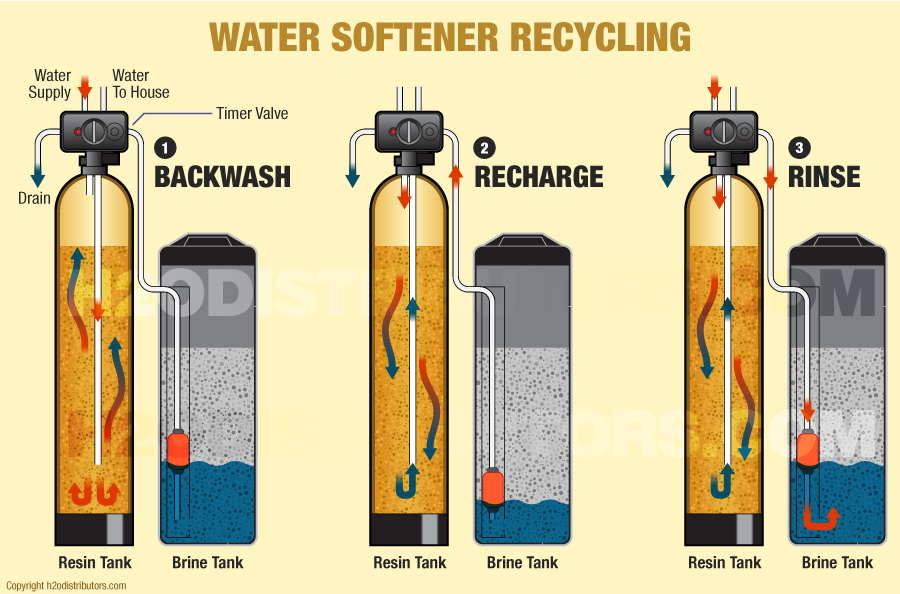
- The backwash phase removes dirt from the mineral tank.
- Recharging the mineral tank with sodium from the brine solution displaces calcium and magnesium, which is then washed down the drain.
- The final phase rinses the mineral tank with fresh water and loads the brine tank so it’s ready for the next cycle.
How Does a Water Softener Work? The Solution Is The Problem
While water is in the ground, it picks up soluble bits of whatever it passes through. While this can mean contamination that makes the water unfit to drink, in many cases it simply means that the water contains minerals found in the earth. Of these, calcium and magnesium are of particular importance because they affect the water’s ability to function in our homes. These minerals make our water hard.
One effect of hard water is that soaps and detergents lose some effectiveness. Instead of dissolving completely, soap combines with the minerals to form a coagulated soap curd. Because less soap is dissolved, more is required. And the sticky insoluble curd hangs around — it clings to the skin and may actually inhibit cleansing. Washed hair seems dull and lifeless.
In the laundry, things aren’t much better. The soap curd can work its way into your clothes as they’re being washed in your automatic washing machine. This can keep dirt trapped in the fibers, and it can stiffen and roughen the fabric.
In addition to affecting the actual washing process, insoluble soap deposits leave spots on everything you wash — from your dishes to the family car — and a soap film will build up in your bath and shower.
Another reason to be concerned about hard water is its effect on your plumbing system. Calcium and magnesium deposits can build up in pipes, reducing flow to taps and appliances. In water heaters, these minerals generate a scale buildup that reduces the efficiency and life of the heater.
The Fix

The solution to the problem is to get rid of the calcium and magnesium. While there are chemical treatments that do this, the most popular answer is a water softener.
The typical water softener is a mechanical appliance that’s plumbed into your home’s water supply system. All water softeners use the same operating principle: They trade the minerals for something else, in most cases sodium. The process is called ion exchange.
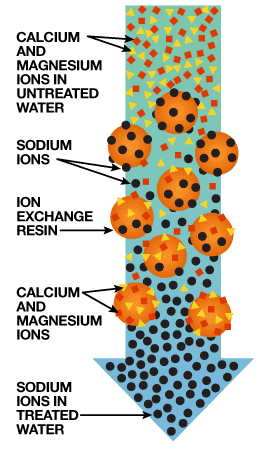
The heart of a water softener is a mineral tank. It’s filled with small polystyrene beads, also known as resin or zeolite. The beads carry a negative charge.
Calcium and magnesium in water both carry positive charges. This means that these minerals will cling to the beads as the hard water passes through the mineral tank. Sodium ions also have positive charges, albeit not as strong as the charge on the calcium and magnesium. When a very strong brine solution is flushed through a tank that has beads already saturated with calcium and magnesium, the sheer volume of the sodium ions is enough to drive the calcium and magnesium ions off the beads. Water softeners have a separate brine tank that uses common salt to create this brine solution.
In normal operation, hard water moves into the mineral tank and the calcium and magnesium ions move to the beads, replacing sodium ions. The sodium ions go into the water. Once the beads are saturated with calcium and magnesium, the unit enters a 3-phase regenerating cycle. First, the backwash phase reverses water flow to flush dirt out of the tank. In the recharge phase, the concentrated sodium-rich salt solution is carried from the brine tank through the mineral tank. The sodium collects on the beads, replacing the calcium and magnesium, which go down the drain. Once this phase is over, the mineral tank is flushed of excess brine and the brine tank is refilled.
The Brains Behind How Water Softeners Work
Most popular water softeners have an automatic regenerating system. The most basic type has an electric timer that flushes and recharges the system on a regular schedule. During recharging, soft water is not available.
A second type of control uses a computer that watches how much water is used. When enough water has passed through the mineral tank to have depleted the beads of sodium, the computer triggers regeneration. These softeners often have reserve resin capacity, so that some soft water will be available during recharging.
A third type of control uses a mechanical water meter to measure water usage and initiate recharging. The advantage of this system is that no electrical components are required and the mineral tank is only recharged when necessary. When it is equipped with two mineral tanks, softened water is always available, even when the unit is recharging.
How Does a Water Softener Work? Judging Water Hardness
Companies that sell water softening equipment generally offer test kits that help you determine the hardness of your water. For commercial testing sources, check your Yellow Pages under “water analysis.”
Water hardness is measured in grains per gallon (GPG) or milligrams per liter (mg/L, equivalent to parts per million, or ppm). Water up to 1 GPG (or 17.1 mg/L) is considered soft, and water from 3.5 – 7.0 GPG (60 to 120 ppm) is considered moderately hard. A water softener’s effectiveness depends on how hard the incoming water is. Water over 100 GPG may not be completely softened.
Health Concerns Regarding Hard Water
Hard water poses no health hazard. On the other hand, the sodium that remains in softened water may be a problem for those on sodium-restricted diets. Other people simply may wish to avoid the slightly salty taste of treated water. In either case you can install a separate water dispenser that bypasses the softener. You also can use potassium chloride instead of salt, although this costs about three to four times more.
The preceding content was taken from PopularMechanics.com.
Our Water Softener Systems














































